Alefiyah Malbari, MD, Discusses Pediatric COVID-19 Vaccination
Children as young as six months are now eligible for the shot
Reviewed by: Alefiyah Malbari, MD
Written by: Lauren Schneider
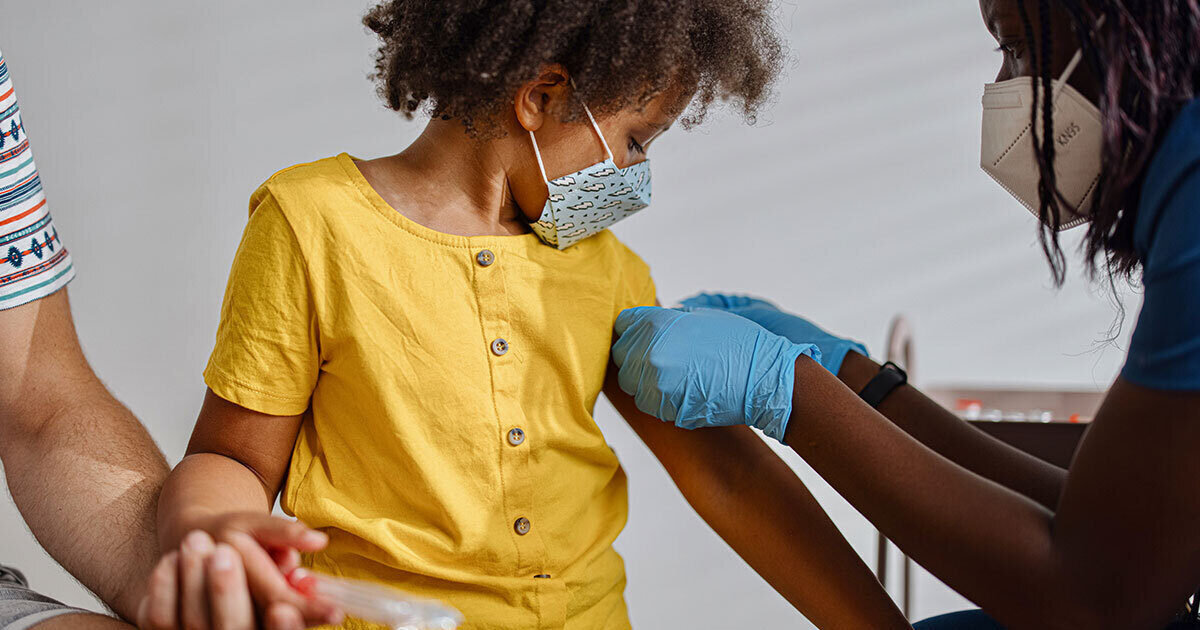
On Saturday, June 18, 2022 the Centers for Disease Control and Prevention (CDC) authorized COVID-19 vaccines for children as young as 6 months.
The CDC’s statement came one day after the US Food and Drug Administration (FDA) announced an emergency use authorization for both the Moderna and Pfizer-BioNTech vaccines in this age group. The Pfizer vaccine will be given as a three-dose series, with three weeks between the first two doses, and an additional eight weeks before the final shot. The Moderna vaccine is a two-dose series in which the second dose is given a month after the first.
Vaccine rollout for children under 5 years of age began on Tuesday, June 21. “We now have a way to protect our youngest children ages six months and up,” says Alefiyah Malbari, MD.
Dr. Malbari is a pediatrician who serves as the Chief of the Division of Ambulatory Pediatrics at The University of Texas at Austin Dell Medical School and the Chief of Dell Children’s Medical Group Pediatrics, a clinical Partnership between Dell Children’s Medical Center and UT Health Austin. She addresses questions that parents may have about vaccinating young children below.
What risk does COVID-19 pose to young children?
“Over two million cases of COVID were reported in kids younger than four years old in the U.S. during the height of the pandemic. Of these cases, about 400 resulted in deaths in this age range,” says Dr. Malbari.
“While for the most part, kids that get COVID have mild or asymptomatic disease, there are cases where kids can get more severe symptoms,” she says. These symptoms include the following:
- Lung infections
- Multisystem Inflammatory Syndrome in Children (MIS-C)
- Long COVID
- Diabetes
According to Dr. Malbari, children in this age range “should definitely get vaccinated” to prevent these complications.
Are certain children at higher risk of complications from COVID-19?
“Children with underlying medical conditions are more susceptible to developing complications from COVID,” says Dr. Malbari. These underlying conditions include, but are not limited to:
- Immunosuppression (whether from a disease that weakens immune system function or from medications like chemotherapy)
- Chronic diseases
- Transplanted organs
How does the vaccine for young children differ from the adult vaccine?
“The Pfizer dose represents one-tenth of the adult dose,” says Dr. Malbari, “and the Moderna dose represents a quarter of the adult dose.”
How effective is the vaccine in protecting young children?
“The data shows very clearly that both the Moderna and Pfizer vaccines are safe and effective,” says Dr. Malbari. Both clinical trials “compared kids who received the vaccine to kids who received a placebo (not the actual vaccine) to determine if the vaccines are safe and effective.”
“Both the Pfizer and Moderna studies found that the number of antibodies made against COVID-19 were comparable to the antibodies made with the higher dose vaccines of the older age group.”
Researchers demonstrated 80% efficacy of the Pfizer vaccine against symptomatic COVID-19 in children. Data from the Moderna trial revealed 50% efficacy in children between the ages of 6 months and 2 years, and 37% efficacy in children 2 to 5 years of age.
Dr. Malbari notes that “the data did not show any cases of severe disease, hospitalization, or death” among children in this age range who received the vaccine.
How was the safety of the vaccine determined in this age group?
Both clinical trials “included close monitoring of all individuals in the study for side effects from the vaccines,” says Dr. Malbari.
What side effects are associated with the vaccine in young children?
“We can expect side effects that are similar to the other childhood vaccines,” says Dr. Malbari. These may include:
- Fever
- Irritability
- Loss of appetite
- Pain or red bump at the site of injection
Drowsiness was the most common side effect observed in children 2-23 months old. The most common side effects reported in 2–5-year-olds were fatigue and pain at the injection site. Dr. Malbari adds that “all of these symptoms tend to resolve by themselves in about 48 hours.”
Where will the vaccine be available for young children?
According to Dr. Malbari, the following locations may offer the pediatric vaccine:
- Your child’s pediatrician
- A primary care provider
- Local health departments and institutions
- Community clinics
- Local pharmacies
“It is important to give your child’s pediatrician or their primary care doctor a call and ask your questions about the vaccine, and they’ll also be able to tell you where you can get your child vaccinated,” she says.
For more information about Dr. Malbari or the Dell Children’s Medical Group Pediatric Mueller, visit here.